J Cardiovasc Thorac Res. 17(1):12-19.
doi: 10.34172/jcvtr.025.33343
Review Article
Effects of Nigella sativa on VCAM-1 and ICAM-1: A systematic review of preclinical and clinical studies
Zeinab Faghfoori Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing, 1, 2 
Zeinab Javadivala Data curation, Methodology, Software, 3
Aida Malek Mahdavi Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, 4, * 
Author information:
1Food Safety Research Center (salt), Semnan University of Medical Sciences, Semnan, Iran
2Department of Nutrition, School of Nutrition and Food Sciences, Semnan University of Medical Sciences, Semnan, Iran
3Department of Health Education & Promotion, Faculty of Health, Tabriz University of Medical Sciences, Tabriz, Iran
4Connective Tissue Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
The objective of present review was to assess all studies about effect of Nigella sativa (N. sativa) on vascular cell and intercellular adhesion molecules-1 (VCAM-1 and ICAM-1) under different situations. Search was performed until May 2024 using Scopus, PubMed, Web of Science, and Google Scholar databases without any restriction and alert services were utilized following the primary search. The references cited in related papers were also evaluated. Nineteen studies including human (n=4), animal (n=11), and in vitro (n=4) were eligible. All in vitro and majority of animal researches were indicative of the favorable effects of N. sativa and thymoquinone in attenuating VCAM-1 and ICAM-1 levels; however, three animal studies did not show any significant effect. Results of clinical trials were conflicting. In two clinical trials, supplementation with N. sativa oil and N. sativa powder led to significant reduction in VCAM-1 levels in coronary artery disease (CAD) and Hashimoto’s thyroiditis patients, whereas no significant change occurred according to the other clinical trial involving subjects with the risk factor for cardiovascular disease (CVD). Furthermore, significant reduction in ICAM-1 levels occurred after N. sativa oil consumption in two clinical trials involving type 2 diabetic and CAD patients, whilst no significant change was noticed in subjects with the risk factor for CVD and Hashimoto’s thyroiditis patients. N. sativa seems beneficial in attenuating VCAM-1 and ICAM-1 levels under different situations; however, additional long-term controlled clinical trials are needed for making concise conclusions about the effect of N. sativa on endothelial dysfunction related biomarkers.
Keywords: Nigella sativa, Vascular cell adhesion molecule-1, Intercellular adhesion molecule-1
Copyright and License Information
© 2025 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
We thank the financial support of the Research Vice-Chancellor of Tabriz University of Medical Sciences, Tabriz, Iran (Grant No: 75625).
Introduction
Endothelial injury has a main function in expansion of vascular events including atherosclerosis, and is characterized by low-grade inflammation that initiates upregulation of cell adhesion molecules.1 Upregulation of various adhesion molecules induces inflammatory pathways and results in a chronic inflammatory situation, if not being managed appropriately.2 Therefore, dysregulation of adhesion molecules can cause different inflammatory and immune-related diseases.2 Adhesion molecules are glycoproteins on cellular surfaces responsible for the connection between cells or between the extracellular matrix and cells.3 During the preliminary phases of atherosclerosis, these molecules enable monocytes to stick to the endothelial cells and migrate under endothelium after attaching to the injured endothelial cells.4 Vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) are amongst the basic contributing adhesion molecules in atherosclerosis.5 VCAM-1 and ICAM-1 are generated on stimulated endothelial cells and atherosclerosis-prone locations leading to the accumulation of inflammatory monocytes in the endothelium.3 Therefore, inhibiting VCAM-1 and ICAM-1 can delay atherosclerosis development and has a significant role in the prevention of atherosclerosis.
Recently, herbal compounds with potential antioxidant and anti-inflammatory activities have been reported to beneficially affect vascular endothelium and endothelial function.6 Thus, considering plants in treating conditions associated with endothelial injury and dysfunction has become an area of interest.7 One such medicinal herb is Nigella sativa (N. sativa).
N. sativa, usually recognized as black cumin or black seed, is a rich source of antioxidants and bioactive agents including polyphenols, flavonoids, saponins, alkaloids, proteins, fatty acids, vitamins, and minerals.8 Thymoquinone (TQ) is the most active component found plentifully in N. sativa, together with its derivatives like thymol and thymohydroquinone.8 N. sativa is a traditional herb, which is a member of the Ranunculaceae family and is frequently consumed in the Middle East, Western Asia, North Africa, and Eastern Europe.8 Antioxidant, anti-inflammatory, hypotensive, hypoglycemic, and hypolipidemic activities have been related to N. sativa.9 In addition, N. sativa and its constituents have shown beneficial effects against diabetes mellitus, hypertension, obesity, dyslipidemia, and metabolic syndrome9, which are linked with vascular dysfunction. Therefore, amelioration in any of these diseases can also improve vascular action.
The effects of N. sativa on adhesion molecules specifically VCAM-1 and ICAM-1 have been studied in recent years; however, some discrepancies exist among the results. In vitro studies10-13 as well as some animal14-20,21 and clinical25-27 studies indicated the beneficial effects of N. sativa in reducing VCAM-1 and ICAM-1, whilst in other animal22-24 and clinical26,28 researches, no effect and/or increased concentrations of these adhesion molecules were reported. Given the inconsistent findings of previous related studies and lack of any comprehensive systematic review encompassing clinical and preclinical studies altogether in this field, present systematic review was conducted to evaluate the effects of N. sativa on VCAM-1 and ICAM-1 under different situations considering data from clinical, animal, and in vitro models.
Methods
Study protocol and search strategy
This study agrees with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.29 The protocol of study was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42024556099.
A literature search was conducted utilizing electronic databases, encompassing Web of Science, Scopus, PubMed, and Google Scholar, up to May 2024. We also utilized search alerts to notice relevant articles after the primary search. The terms utilized to search within titles, abstracts, and keywords were: “Nigella sativa”, “Nigella sativas”, “sativa, Nigella”, “Cumin, Black”, “Black Cumin”, “Black Cumins”, “Cumins, Black”, Kalonji, Kalonjus, “thymoquinone”, dihydrothymoquinone, “2-isopropyl-5-methylbenzoquinone”, “2-methyl-5-isopropyl-p-benzoquinone”, “Vascular Cell Adhesion Molecule-1”, “Vascular Cell Adhesion Molecule 1”, “Inducible Cell Adhesion Molecule 110”, INCAM-110, “Vascular Cell Adhesion Molecule”, VCAM-1, “CD106 Antigen”, “Antigen, CD106”, “Antigens, CD106”, “CD106 Antigens”, “Intercellular Adhesion Molecule-1”, “Intercellular Adhesion Molecule 1”, ICAM-1, “CD54 Antigen”, “Antigen, CD54”, “Antigens, CD54”, “CD54 Antigens”. We utilized both MeSH terms and text words without inflicting any restrictions on language or publication date. Two researchers separately performed the search and screening activities. Duplicates were recognized and eliminated. The references of relevant papers were assessed to uncover related studies. A consensus existed between the two authors on article selection, and any potential discrepancies were addressed and resolved by the third researcher.
Inclusion and exclusion criteria
This scientific review adhered to strict criteria to choose relevant studies on the effects of N. sativa on VCAM-1 and ICAM-1. Studies were included if they assessed the effects of N. sativa compared with a control arm and if the full text of the research was accessible. Conversely, studies excluded from the review were those that did not directly assess the impact of N. sativa on VCAM-1 and ICAM-1, such as review articles, book chapters, or studies on the combined impact of N. sativa with other compounds. Additionally, any research not published in a peer-reviewed journal or with limited access to the full text was excluded.
Data extraction
After using inclusion and exclusion criteria, 19 studies were selected for further analysis (Figure 1). Data pertaining to the first author’s last name, issue year, sample characteristics, type and dose of N. sativa administered, period of intervention, and reported outcomes were gathered from the selected studies. A comprehensive overview of these studies is presented in Tables 1 to 3.
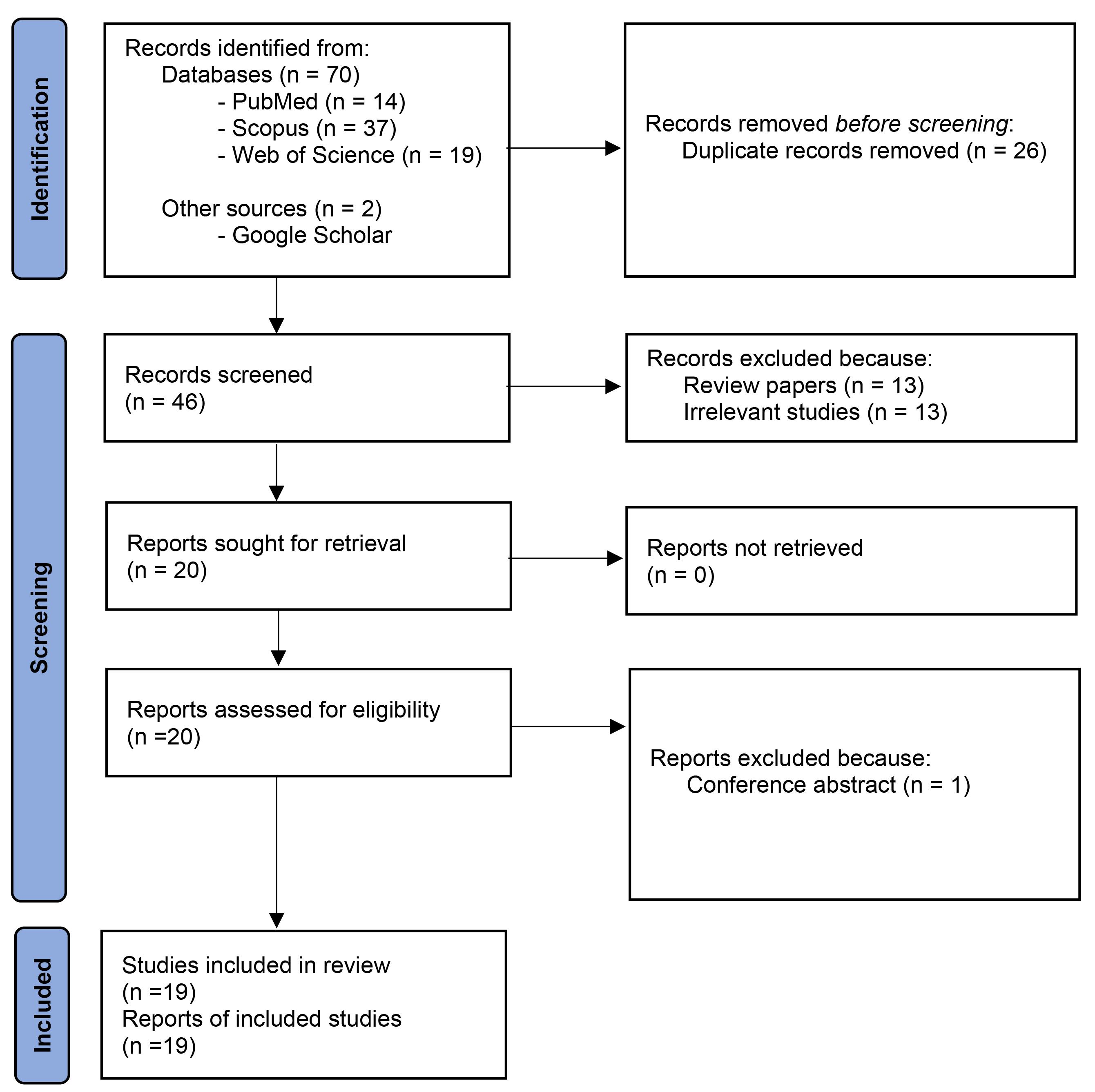
Figure 1.
The flowchart of screening and selecting articles
.
The flowchart of screening and selecting articles
Table 1.
Characteristics of included in vitro studies
|
Author
|
Population
|
Intervention
|
Dose
|
Duration
|
Findings
|
| Khan et al10 |
Human coronary artery endothelial cells |
Nigella sativa oil
and Thymoquinone |
55, 110, 220, and 440 µg/ml
4.5, 9.0, 18.0, and 36.0 µm |
24 hours |
Significant dose-dependent reduction in VCAM-1 and ICAM-1 gene and protein expressions |
| Huwait et al 11 |
Human THP-1 macrophages |
Thymoquinone |
2.5, 5, 7.5, and 10 µM |
24 hours |
Significant decrease in mRNA expression of ICAM-1 |
| Umar et al12 |
RA synovial fibroblasts |
Thymoquinone |
1–5 μM |
2 hours |
Significant dose-dependent reduction in ICAM-1 and VCAM-1 expression |
| Xuan et al 13 |
Mouse dendritic cells |
Thymoquinone |
1, 5, 10, and 20 μM |
24 hours |
Significant inhibition of ICAM-1 expression |
VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intracellular adhesion molecule-1; RA, rheumatoid arthritis.
Table 2.
Characteristics of included animal studies
|
Author
|
Population
|
Intervention
|
Dose
|
Route
|
Duration
|
Findings
|
| Triastuti et al 14 |
Rats with sub-chronical cigarette smoke exposure (n = 50) |
Ethanolic extract of Nigella sativa |
0.3, 0.6, and 1.2 g/kg/day |
Gastric tube |
4 weeks |
Significant dose-dependent reduction in VCAM-1 expression compared to the exposed control group |
| Ashour et al15 |
Rats with renal ischemia-reperfusion injury (n = 48) |
Thymoquinone |
10 mg/kg/day |
Intravenous |
10 days |
Significant reduction in renal VCAM-1 level compared to the renal ischemia-reperfusion injury control group |
| Ashour et al16 |
Rats with remote hepatic injury post-renal reperfusion (n = 30) |
Thymoquinone |
10 mg/kg/day |
Intravenous |
10 days |
Significant reduction in liver VCAM-1 level compared to the reperfusion injury control group |
| Alzohairy et al 17 |
Rats with Benzo(a)pyrene-induced
lung injury (n = 32) |
Thymoquinone |
50 mg/kg/day |
Oral |
8 weeks |
Significant reduction in ICAM-1 level compared to the lung injury control group |
| Abbasnezhad et al 18 |
Streptozotocin-induced diabetic rats (n = 70) |
Hydroalcoholic extract of Nigella sativa seed |
100, 200, and 400 mg/kg/day |
Gavage |
6 weeks |
Significant reduction in VCAM-1 mRNA expression compared to the diabetic control group |
| Alhusaini et al19 |
Rats with sodium fluoride-induced acute renal injury (n = 50) |
Thymoquinone |
10 mg/kg/day |
Oral |
4 weeks |
Significant reduction in protein expression of VCAM compared to the acute renal injury control group |
| Elmorsy et al 20 |
Experimentally-induced atherosclerosis rabbits (n = 30) |
Nigella sativa powder |
150 mg/kg/day |
Gavage |
20 weeks
&
8 weeks |
Significant reduction in serum VCAM-1 and ICAM-1 levels compared to the atherosclerosis control group |
| Saleh and El-Abhar21 |
Shistosoma mansoni infected mice (50-55) |
Thymoquinone |
5 and 10 mg/kg/day |
Oral |
2 weeks |
Significant reduction in ICAM-1 expression in Kupffer and inflammatory cells compared with the infected control group |
| Al-Asoom et al22 |
Rats with physiological cardiac hypertrophy (n = 45) |
Nigella sativa suspension |
800 mg/kg/day |
Oral |
8 weeks |
No significant change in serum ICAM-1 level compared with the control group |
| Al Wafai et al23 |
Streptozotocin-induced diabetic rats (n = 150) |
Nigella sativa aqueous extractand Nigella sativa oil and Thymoquinone |
2 mL/kg/day
0.2 mL/kg/day
5 mg/kg/day |
Intra-peritoneal injection |
6 days/week |
No significant change in ICAM-1 mRNA expression in pancreatic tissue compared with the diabetic control group |
| Finlay et al24 |
Wild-type (WT), Neu4 KO (Neu4 knockout), and Neu1-CathA KD (Neu1 deficient and cathepsin A
deficient) mice (n = 15) |
Thymoquinone |
2.5 mg |
Intra-peritoneal injection |
- |
Significant increase in serum ICAM-1 compared with the control group |
VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intracellular adhesion molecule-1.
Table 3.
Characteristics of included human studies
|
Author
|
Population
|
Intervention
|
Dose
|
Route
|
Duration
|
Findings
|
| Tavakoli-Rouzbehani et al 25 |
Coronary artery disease patients (n = 60) |
Nigella sativa oil |
2 g/day |
Oral |
8 weeks |
Significant decrease in serum VCAM-1 and ICAM-1 levels compared with the coronary artery disease control group |
| Abbasalizad Farhangi & Tajmiri26 |
Hashimoto's thyroiditis patients (n = 40) |
Nigella sativa powder |
2 g/day |
Oral |
8 weeks |
(1) Significant decrease in serum VCAM-1 levels compared with the control group
(2) No significant change in serum ICAM-1 levels compared with the Hashimoto's thyroiditis control group |
| Elgarf et al27 |
Type 2 diabetic patients (n = 56) |
Nigella sativa oil |
1.8 g/day |
Oral |
12 weeks |
Significant decrease in serum ICAM-1 levels compared with the diabetic control group |
| Emamat et al28 |
Subjects with at least one risk factor for cardiovascular disease (n = 50) |
Nigella sativa oil |
1 g/day |
Oral |
8 weeks |
No significant change in plasma VCAM-1 and ICAM-1 levels compared with the control group |
VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intracellular adhesion molecule-1.
Evaluation of bias risk
The risk of bias (RoB) in the included clinical, animal, and in vitro researches was assessed using the Cochrane Collaborationʼs tool, the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE’s) RoB tool, and the Checklist for Reporting In vitro Studies (CRIS) instruction, respectively. The SYRCLEʼs RoB tool relies on the Cochrane Rob tool and both of the tools have six domains, and every domain was judged as possessing a low, unclear, or high risk.8
Results
Study Selection
Overall 72 papers were detected primarily (Figure 1). After removing duplicates, 46 papers were screened by their titles and abstracts. Finally, out of 20 potentially related papers, one paper was deleted due to abstract in conference. Finally, 19 papers including human (n = 4), animal (n = 11), and in vitro (n = 4) researches were kept. Tables 1 to 3 show details of the researches.
Characteristics of the included studies
The primary characteristics of the included studies are outlined in Tables 1 to 3. Studies were conducted in the following countries: Malaysia,10 Saudi Arabia,11,16,17,19,22 USA,12 Germany,13 Indonesia,14 Egypt,15,20,21,27 Iran,18,25,26,28 Lebanon,23 Canada.24 The included in vitro studies used TQ with different doses, ranging from 1 to 36 μM. In one in vitro study,10 N. sativa oil with doses of 55, 110, 220, and 440 µg/ml was also used. The duration of treatment was 24 hours in three in vitro studies10,11,13 and 2 hours in one in vitro study.12 A variety of in vitro models were used including human coronary artery endothelial cells,10 human THP-1 macrophages,11 RA synovial fibroblasts,12 and mouse dendritic cells.13 Moreover, the included animal studies varied in length, ranging from 6 days/week to 20 weeks and route of administration including oral, gastric tube, gavage, intravenous, and intra-peritoneal injection. Ethanolic extract of N. sativa with doses of 0.3, 0.6, and 1.2 g/kg/day and hydro-alcoholic extract of N. sativa with doses of 100, 200, and 400 mg/kg/day were used in two animal studies,14,18 respectively. In the other animal studies, N. sativa powder with a dose of 150 mg/kg/day,20 TQ with doses of 5 to 50 mg/kg/day15-17,19,21,23 and 2.5 mg,24 N. sativa suspension with a dose of 800 mg/kg/day,22 N. sativa aqueous extract with a dose of 2 mL/kg/day as well as N. sativa oil with a dose of 0.2 mL/kg/day23 were used. A variety of animal models were studied in these animal investigations including rats with sub-chronical cigarette smoke exposure,14 rats with renal ischemia-reperfusion injury,15 rats with remote hepatic injury post-renal reperfusion,16 rats with Benzo(a)pyrene-induced lung injury,17 streptozotocin-induced diabetic rats,18,23 rats with sodium fluoride-induced acute renal injury,19 experimentally-induced atherosclerosis rabbits,20 Shistosoma mansoni-infected mice,21 rats with physiological cardiac hypertrophy22 as well as Wild-type, Neu4 knockout, and Neu1 deficient and cathepsin A deficient mice.24 Furthermore, the included clinical trials varied in length, ranging from 8 to 12 weeks. N. sativa oil25,27,28 were used in three studies with doses from 1 to 2 g/day. In the other clinical trial,26 N. sativa powder was used with dose of 2 g/day. A variety of patient populations were studied in the included clinical trials. This includes subjects with coronary artery disease,25 Hashimoto’s thyroiditis,26 type 2 diabetes,27 and subjects with at least one cardiovascular disease (CVD) risk factor28.
In vitro investigations
Four in vitro studies were eligible (Table 1). Khan et al10 indicated that 55, 110, 220, and 440 µg/ml N. sativa oil and 4.5, 9.0, 18.0, and 36.0 µm TQ for 24 hours led to remarkable dose-dependent decrease in VCAM-1 and ICAM-1 gene and protein expressions in human coronary artery endothelial cells. In another study, Huwait et al11 stated that 2.5, 5, 7.5, and 10 µM TQ for 24 hours significantly decreased mRNA expression of ICAM-1 in human THP-1 macrophages. Furthermore, Umar et al12 showed that 1–5 μM TQ for 2 hours caused significant dose-dependent reduction in expression of ICAM-1 and VCAM-1 in RA synovial fibroblasts. Xuan et al13 also demonstrated that 1, 5, 10, and 20 μM TQ for 24 hours significantly inhibited ICAM-1 expression in mouse dendritic cells.
Animal investigations
Eleven animal studies were eligible (Table 2).Triastuti et al14 indicated that 0.3, 0.6, and 1.2 g/kg/day ethanolic extract of N. sativa for 4 weeks dose-dependently reduced VCAM-1 expression in rats with sub-chronical cigarette smoke exposure compared to the cigarette smoke-exposed control rats. Ashour et al15,16 showed that 10 mg/kg/day TQ for 10 days considerably reduced renal and liver VCAM-1 level in rats with renal ischemia-reperfusion injury and in rats with remote hepatic injury post-renal reperfusion, respectively compared with the reperfusion injury controls. Moreover, Alzohairy et al17 reported that oral consumption of 50 mg/kg/day TQ for 8 weeks significantly alleviated ICAM-1 level in rats with Benzo(a)pyrene-induced lung injury compared to the lung injury control ones. Furthermore, Abbasnezhad et al18 reported that 100, 200, and 400 mg/kg/day hydro-alcoholic extract of N. sativa seed for 6 weeks considerably reduced VCAM-1 mRNA expression in streptozotocin-induced diabetic rats compared with the diabetic control animals. Alhusaini et al19 demonstrated that 10 mg/kg/day TQ for 4 weeks significantly reduced protein expression of VCAM in rats with sodium fluoride-induced acute renal injury compared to the acute renal injury control group. Elmorsy et al20 found that 150 mg/kg/day N. sativa powder led to remarkable reduction in serum VCAM-1 and ICAM-1 levels in experimentally-induced atherosclerosis rabbits in comparison to the atherosclerosis control arm. In addition, Saleh and El-Abhar21 suggested that 5 and 10 mg/kg/day TQ for 2 weeks significantly reduced ICAM-1 expression in Kupffer and inflammatory cells in Shistosoma mansoni-infected mice compared with the infected controls. Another research by Al-Asoom et al22 showed that 800 mg/kg/day N. sativa suspension for 8 weeks did not cause significant change in serum ICAM-1 level in rats with physiological cardiac hypertrophy compared with the control arm. In another study, Al Wafai et al23 indicated that 2 mL/kg/day N. sativa aqueous extract, 0.2 mL/kg/day N. sativa oil, and 5 mg/kg/day TQ for 6 days/week did not significantly change in ICAM-1 mRNA expression in pancreatic tissue in streptozotocin-induced diabetic rats in comparison with the diabetic controls. Finlay et al24 suggested that 2.5 mg TQ significantly increased serum ICAM-1 in Wild-type, Neu4 knockout, and Neu1 deficient and cathepsin A deficient mice compared to the control mice.
Clinical investigations
Four clinical studies were eligible (Table 3).According to Tavakoli-Rouzbehani et al25 reported that 2 g/day N. sativa oil for 8 weeks considerably decreased serum VCAM-1 and ICAM-1 in coronary artery disease patients compared with the control patients. Also, Abbasalizad Farhangi and Tajmiri26 reported that consuming 2 g/day N. sativa powder for 8 weeks caused significant decrease in serum VCAM-1 levels, whereas did not significantly alter serum ICAM-1 levels in Hashimoto’s thyroiditis patients compared with the controls. Furthermore, Elgarf et al27 indicated that oral consumption of 1.8 g/day N. sativa oil for 12 weeks remarkably decreased serum ICAM-1 levels in patients with type 2 diabetes compared with the diabetic control subjects. In another study, Emamat et al,28 consuming 1 g/day N. sativa oil for 8 weeks did not remarkably change plasma VCAM-1 and ICAM-1 levels in subjects with at least one CVD risk factor compared to the control subjects.
Methodological quality
There was an unclear risk for selection bias (absence of data about the randomization procedure: n = 15); detection bias (masking of outcome evaluation: n = 14); performance bias (masking of the researcher regarding intervention: n = 15) and attrition bias (n = 15). Reporting bias: n = 19 and baseline details of animal and in vitro samples: n = 15 was low. A report for risk of bias was noted in Figure 2.
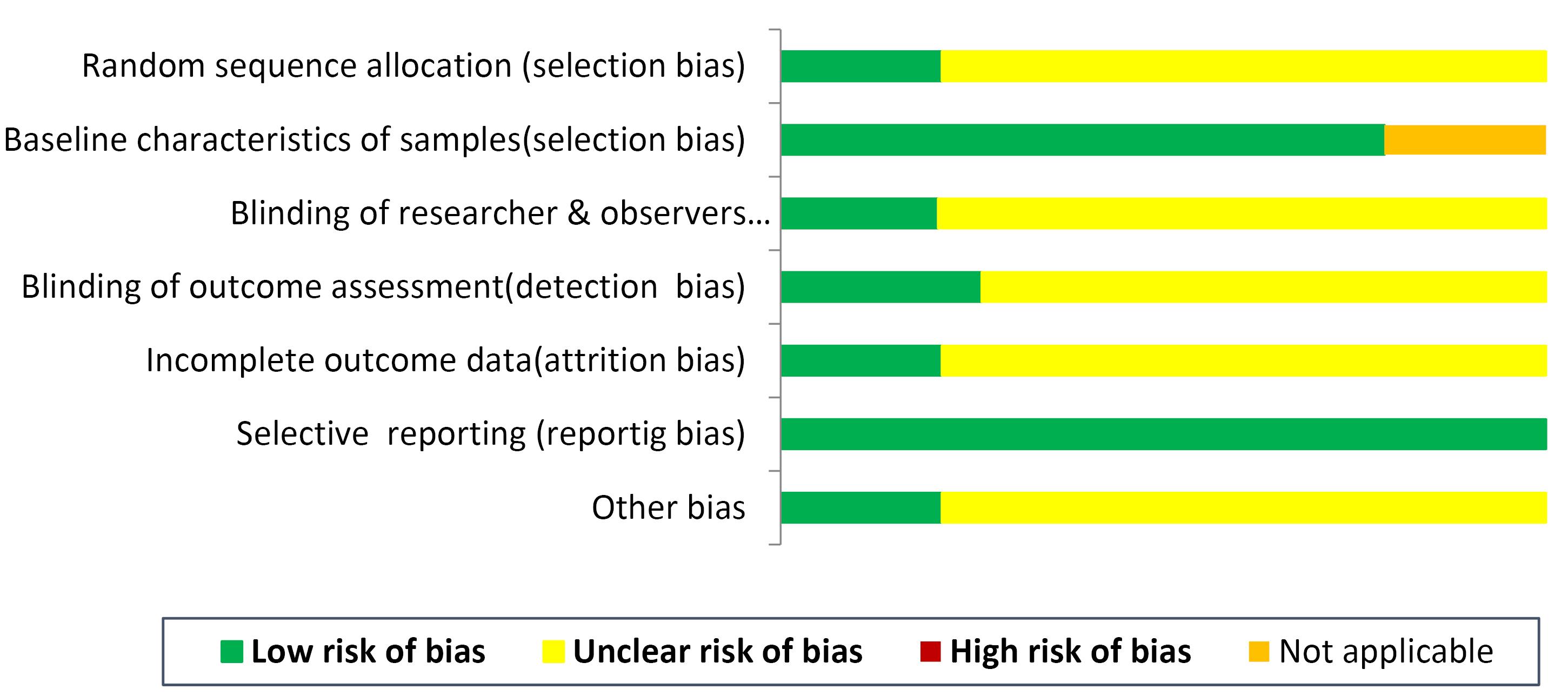
Figure 2.
The risk of bias assessment across the studies (n = 19)
.
The risk of bias assessment across the studies (n = 19)
Discussion
To the authors’ knowledge, this systematic review is the first assessing the existing literature regarding the effect of N. sativa on VCAM-1 and ICAM-1 under different situations considering data from clinical, animal, and in vitro researches. All in vitro10-13 and almost all animal14-20,21 investigations demonstrated the favorable effects of N. sativa and TQ in attenuating VCAM-1 and ICAM-1 levels; however, three animal studies22-24 did not demonstrate any significant effect of N. sativa on VCAM-1 and ICAM-1 levels. Results of clinical trials regarding the effect of N. sativa on VCAM-1 and ICAM-1 were also conflicting.25-28 Some clinical trials25,26 showed that N. sativa oil and N. sativa powder supplementation led to significant reduction in VCAM-1 levels, whereas no significant change was observed in VCAM-1 levels according to Emamat et al28 research. Furthermore, significant decrease in ICAM-1 was observed after N. sativa oil consumption in studies by Tavakoli-Rouzbehani et al25 and Elgarf et al,27 while no significant alteration was noticed in ICAM-1 according to some other clinical trials.26,28 Variations in study designs, characteristics of study samples, baseline concentrations of VCAM-1 and ICAM-1, preparation and administration methods, dosage, duration, and bioavailability rate of N. sativa and/or its components seem to be responsible for discrepancies among researches. The N. sativa or its active constituents were directly utilized in animal models, whereas in clinical trials, N. sativa powder and oil were prescribed in the form of capsule. Furthermore, level of TQ, the principle compound of N. sativa, varies according to the preparation procedure and storage condition of products of N. sativa, which can lead to meaningful variety in bioactive compounds among studies. Altogether, this review indicated that adhesion molecules such as VCAM-1 and ICAM-1 had a meaningfully descending orientation after using N. sativa and/or TQ. This study was consistent with Mohebbatia and Abbasnezhad30 study, which concluded that N. sativa and TQ had a protective impact on endothelial dysfunction initiated by diabetes. However, a newly published meta-analysis of controlled trials concluded that supplementation with N. sativa did not have a meaningful impact on the endothelial function responses including ICAM-1 and VCAM-1 in subjects with CVD or the risks of CVD, highlighting a disagreement that emphasizes the intricacy of N. sativa effects on endothelial function and the need for further research.31 These authors also noted that the pooled data were severely heterogeneous, which affected their results.31
As mentioned earlier, upregulation of cellular adhesion molecules like VCAM-1 and ICAM-1 has a close relationship with endothelial injury and dysfunction, which is the primitive phase of atherosclerosis. Therefore, VCAM-1 and ICAM-1 can be considered as predictors of CVD events and closely related to the atherosclerosis development.32 N. sativa is a well-tolerated and safe herb and most of its helpful medicinal properties are due to volatile oil, of that TQ is a main component.33 Different mechanisms are indicated for potential ameliorative effect of N. sativa or its bioactive agent TQ on adhesion molecules. N. sativa has a lipid-lowering property, which is due to the suppression of de novo cholesterol synthesis or induction of bile acid excretion. Therefore, N. sativa through modulating lipid profile may inhibit VCAM-1 gene expression, reduce atherosclerotic plaque generation, and promote endothelial integrity.34-36 Moreover, N. sativa may attenuate VCAM-1 and ICAM-1 concentrations via decreasing the expression of oxidized low-density lipoprotein receptor-1 (LOX-1), which is the principle receptor in endothelial cells for oxidized low-density lipoprotein (LDL).18 Oxidized LDL uptake by LOX-1 reduces endothelial nitric oxide synthase (eNOS) expression and nitric oxide production and further induces adhesion molecule expression, thereby leading to endothelial dysfunction.37,38 Moreover, oxidized LDL binding to LOX-1 contributes to oxidative stress and superoxide generation and nuclear factor-kappa B (NF-κB) induction.39 The other mechanism is that N. sativa may decrease adhesion molecules’ expression via reducing reactive oxygen species (ROS) generation and endothelial cell injury by its direct antioxidant feature.40 A part of the antioxidant activity of N. sativa is attributed to its TQ content. This compound increases the bioavailability of nitric oxide through stimulating the activity of the enzyme eNOS, which is important for vasodilation and vascular health.41,42 The antioxidant function of N. sativa has also been demonstrated via increasing the antioxidant enzymes.22 The impaired antioxidant system can increase ROS and pro-inflammatory mediators leading to elevated adhesion molecule expression and atherosclerosis development.43 Additionally, TQ in N. sativa can lower adhesion molecules’ expression via suppressing pro-inflammatory cytokines or enzymes such as monocyte chemoattractant protein-1, tumor necrosis factor-α, interleukin (IL)-1β, IL-8, IL-6, nuclear factor-kappa B, and cyclooxygenase-2, which lead to anti-inflammatory effect in the body.44
A limitation of the current review was the limited number of clinical studies, whereas the number of preclinical investigations was acceptable. The strength of current review was that all preclinical and clinical investigations were gathered in a systematic manner without any restriction regarding language and/or publication date.
Conclusion
In conclusion, N. sativa seems beneficial in attenuating VCAM-1 and ICAM-1 levels under different situations. This systematic review was just a description of accessible literature about the influence of N. sativa on VCAM-1 and ICAM-1 together with potential mechanisms and indicated the need for additional long-term controlled clinical trials to make concise conclusions about the effect of N. sativa on endothelial dysfunction related biomarkers.
Competing Interests
The authors declare no conflict of interest.
Ethical Approval
This study was approved by the Ethics Committees of Tabriz University of Medical Sciences, Tabriz, Iran.
References
- Moriya J. Critical roles of inflammation in atherosclerosis. J Cardiol 2019; 73(1):22-7. doi: 10.1016/j.jjcc.2018.05.010 [Crossref] [ Google Scholar]
- Nowak K, Gumkowska-Sroka O, Kotyla P. Adhesion molecules: a way to understand lupus. Reumatologia 2022; 60(2):133-41. doi: 10.5114/reum.2022.115664 [Crossref] [ Google Scholar]
- Hu Q, Saleem K, Pandey J, Charania AN, Zhou Y, He C. Cell adhesion molecules in fibrotic diseases. Biomedicines 2023; 11(7):1995. doi: 10.3390/biomedicines11071995 [Crossref] [ Google Scholar]
- Harjunpää H, Llort Asens M, Guenther C, Fagerholm SC. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front Immunol 2019; 10:1078. doi: 10.3389/fimmu.2019.01078 [Crossref] [ Google Scholar]
- Ebrahimi F, Ghazimoradi MM, Fatima G, Bahramsoltani R. Citrus flavonoids and adhesion molecules: potential role in the management of atherosclerosis. Heliyon 2023; 9(11):e21849. doi: 10.1016/j.heliyon.2023.e21849 [Crossref] [ Google Scholar]
- Varadharaj S, Kelly OJ, Khayat RN, Kumar PS, Ahmed N, Zweier JL. Role of dietary antioxidants in the preservation of vascular function and the modulation of health and disease. Front Cardiovasc Med 2017; 4:64. doi: 10.3389/fcvm.2017.00064 [Crossref] [ Google Scholar]
- Asgary S, Karimi R, Joshi T, Kilpatrick KL, Moradi S, Samimi Z. Effect of pomegranate juice on vascular adhesion factors: a systematic review and meta-analysis. Phytomedicine 2021; 80:153359. doi: 10.1016/j.phymed.2020.153359 [Crossref] [ Google Scholar]
- Khabbazi A, Javadivala Z, Seyedsadjadi N, Malek Mahdavi A. A systematic review of the potential effects of Nigella sativa on rheumatoid arthritis. Planta Med 2020; 86(7):457-69. doi: 10.1055/a-1143-8521 [Crossref] [ Google Scholar]
- Rahmani A, Niknafs B, Naseri M, Nouri M, Tarighat-Esfanjani A. Effect of Nigella sativa oil on oxidative stress, inflammatory, and glycemic control indices in diabetic hemodialysis patients: a randomized double-blind, controlled trial. Evid Based Complement Alternat Med 2022; 2022:2753294. doi: 10.1155/2022/2753294 [Crossref] [ Google Scholar]
- Yuhainis Firus Khan A, Mohtar F, Rahman TA, Muid SA, Froemming GR, Nawawi H. In vitro study of Nigella sativa and thymoquinone activity on endothelial activation and monocyte adhesion. J Appl Biomed 2023; 21(2):73-9. doi: 10.32725/jab.2023.006 [Crossref] [ Google Scholar]
- Huwait E, Al-Gharawi N, Al-Ghamdi MA, Gari M, Prola A, Natesan Pushparaj P. Thymoquinone (TQ) inhibits inflammation and migration of THP-1 macrophages: mechanistic insights into the prevention of atherosclerosis using in-vitro and in-silico analysis. Curr Issues Mol Biol 2022; 44(4):1740-53. doi: 10.3390/cimb44040120 [Crossref] [ Google Scholar]
- Umar S, Hedaya O, Singh AK, Ahmed S. Thymoquinone inhibits TNF-α-induced inflammation and cell adhesion in rheumatoid arthritis synovial fibroblasts by ASK1 regulation. Toxicol Appl Pharmacol 2015; 287(3):299-305. doi: 10.1016/j.taap.2015.06.017 [Crossref] [ Google Scholar]
- Xuan NT, Shumilina E, Qadri SM, Götz F, Lang F. Effect of thymoquinone on mouse dendritic cells. Cell Physiol Biochem 2010; 25(2-3):307-14. doi: 10.1159/000276563 [Crossref] [ Google Scholar]
- Triastuti F, Ardiana M, Andrianto A, Hermawan HO, Ghassani DN. Black cumin inhibits pro-atherogenic changes and reduces aortic intima media thickness in rats with sub-chronical cigarette smoke exposure. Trop J Nat Prod Res 2022; 6(12):2000-6. doi: 10.26538/tjnpr/v6i12.17 [Crossref] [ Google Scholar]
- Ashour H, Rashed L, Elkordy MA, Abdelwahed OM, Ashour H, Rashed L. Thymoquinone ameliorates acute kidney injury induced by renal ischemia-reperfusion. Int J Morphol 2021; 39(2):469-76. doi: 10.4067/s0717-95022021000200469 [Crossref] [ Google Scholar]
- Ashour H, Rashed L, Elkordy MA, Abdelwahed OM. Remote liver injury following acute renal ischaemia-reperfusion: involvement of circulating exosomal miR-687 and regulation by thymoquinone. Exp Physiol 2021; 106(11):2262-75. doi: 10.1113/ep089765 [Crossref] [ Google Scholar]
- Alzohairy MA, Khan AA, Alsahli MA, Almatroodi SA, Rahmani AH. Protective effects of thymoquinone, an active compound of Nigella sativa, on rats with benzo(a)pyrene-induced lung injury through regulation of oxidative stress and inflammation. Molecules 2021; 26(11):3218. doi: 10.3390/molecules26113218 [Crossref] [ Google Scholar]
- Abbasnezhad A, Niazmand S, Mahmoudabady M, Rezaee SA, Soukhtanloo M, Mosallanejad R. Nigella sativa L seed regulated eNOS, VCAM-1 and LOX-1 genes expression and improved vasoreactivity in aorta of diabetic rat. J Ethnopharmacol 2019; 228:142-7. doi: 10.1016/j.jep.2018.09.021 [Crossref] [ Google Scholar]
- Alhusaini AM, Faddah LM, El Orabi NF, Hasan IH. Role of some natural antioxidants in the modulation of some proteins expressions against sodium fluoride-induced renal injury. Biomed Res Int 2018; 2018:5614803. doi: 10.1155/2018/5614803 [Crossref] [ Google Scholar]
- Elmorsy EA, Elesawy BH, El-Baz HA. Effect of black cumin (Nigella sativa) powder on serum lipid profile, malondialdhydes, nitrites, SICAM-1 and SVCAM-1 in experimentally induced atherosclerosis. Int J Pharm Pharm Sci 2015; 7(3):412-7. [ Google Scholar]
- Saleh S, Mahmoud M, El-Abhar H, Omran ZS. Evaluation of the antischistosomal effect of thymoquinone in murine Shistosomamansoni: influence on nitric oxide, ICAM-1 and collagen. Kasr Al Aini Med J 2005; 11(1):117-26. [ Google Scholar]
- Al-Asoom LI, Al-Shaikh BA, Bamosa AO, El-Bahai MN. Effect of Nigella sativa supplementation to exercise training in a novel model of physiological cardiac hypertrophy. Cardiovasc Toxicol 2014; 14(3):243-50. doi: 10.1007/s12012-014-9248-0 [Crossref] [ Google Scholar]
- Al Wafai RJ. Nigella sativa and thymoquinone suppress cyclooxygenase-2 and oxidative stress in pancreatic tissue of streptozotocin-induced diabetic rats. Pancreas 2013; 42(5):841-9. doi: 10.1097/MPA.0b013e318279ac1c [Crossref] [ Google Scholar]
- Finlay TM, Abdulkhalek S, Gilmour A, Guzzo C, Jayanth P, Amith SR. Thymoquinone-induced Neu4 sialidase activates NFκB in macrophage cells and pro-inflammatory cytokines in vivo. Glycoconj J 2010; 27(6):583-600. doi: 10.1007/s10719-010-9302-5 [Crossref] [ Google Scholar]
- Tavakoli-Rouzbehani OM, Abbasnezhad M, Kheirouri S, Alizadeh M. Efficacy of Nigella sativa oil on endothelial function and atherogenic indices in patients with coronary artery diseases: a randomized, double-blind, placebo-control clinical trial. Phytother Res 2022; 36(12):4516-26. doi: 10.1002/ptr.7568 [Crossref] [ Google Scholar]
- Abbasalizad Farhangi M, Tajmiri S. The effects of powdered black cumin seeds on markers of oxidative stress, intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 in patients with Hashimoto’s thyroiditis. Clin Nutr ESPEN 2020; 37:207-12. doi: 10.1016/j.clnesp.2020.02.015 [Crossref] [ Google Scholar]
- Elgarf AT, Aboromia MM, Sabri NA, Shaheen SM. Amelioration of endothelial dysfunction and inflammation in type 2 diabetic patients after black seed oil supplementation. Arch Pharm Sci Ain Shams Univ 2021; 5(2):317-30. doi: 10.21608/aps.2021.108852.1074 [Crossref] [ Google Scholar]
- Emamat H, Mousavi SH, Kargar Shouraki J, Hazrati E, Mirghazanfari SM, Samizadeh E. The effect of Nigella sativa oil on vascular dysfunction assessed by flow-mediated dilation and vascular-related biomarkers in subject with cardiovascular disease risk factors: a randomized controlled trial. Phytother Res 2022; 36(5):2236-45. doi: 10.1002/ptr.7441 [Crossref] [ Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4(1):1. doi: 10.1186/2046-4053-4-1 [Crossref] [ Google Scholar]
- Mohebbati R, Abbasnezhad A. Effects of Nigella sativa on endothelial dysfunction in diabetes mellitus: a review. J Ethnopharmacol 2020; 252:112585. doi: 10.1016/j.jep.2020.112585 [Crossref] [ Google Scholar]
- Ali M, Tantawi M, Kamel AH, Tameemi ZF, Rezk AA, Abdo M. Endothelial function responses to Nigella sativa (black seed) supplementation: a systematic review and meta-analysis of randomized controlled trials. Cureus 2024; 16(5):e61047. doi: 10.7759/cureus.61047 [Crossref] [ Google Scholar]
- Kaur R, Singh V, Kumari P, Singh R, Chopra H, Emran TB. Novel insights on the role of VCAM-1 and ICAM-1: potential biomarkers for cardiovascular diseases. Ann Med Surg (Lond) 2022; 84:104802. doi: 10.1016/j.amsu.2022.104802 [Crossref] [ Google Scholar]
- Derosa G, D’Angelo A, Maffioli P, Cucinella L, Nappi RE. The use of Nigella sativa in cardiometabolic diseases. Biomedicines 2024; 12(2):405. doi: 10.3390/biomedicines12020405 [Crossref] [ Google Scholar]
- Shabana A, El-Menyar A, Asim M, Al-Azzeh H, Al Thani H. Cardiovascular benefits of black cumin (Nigella sativa). Cardiovasc Toxicol 2013; 13(1):9-21. doi: 10.1007/s12012-012-9181-z [Crossref] [ Google Scholar]
- Daryabeygi-Khotbehsara R, Golzarand M, Ghaffari MP, Djafarian K. Nigella sativa improves glucose homeostasis and serum lipids in type 2 diabetes: a systematic review and meta-analysis. Complement Ther Med 2017; 35:6-13. doi: 10.1016/j.ctim.2017.08.016 [Crossref] [ Google Scholar]
- Sahebkar A, Beccuti G, Simental-Mendía LE, Nobili V, Bo S. Nigella sativa (black seed) effects on plasma lipid concentrations in humans: a systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol Res 2016; 106:37-50. doi: 10.1016/j.phrs.2016.02.008 [Crossref] [ Google Scholar]
- Mehta JL, Li DY, Chen HJ, Joseph J, Romeo F. Inhibition of LOX-1 by statins may relate to upregulation of eNOS. Biochem Biophys Res Commun 2001; 289(4):857-61. doi: 10.1006/bbrc.2001.6070 [Crossref] [ Google Scholar]
- Chen H, Li D, Saldeen T, Mehta JL. Transforming growth factor-beta(1) modulates oxidatively modified LDL-induced expression of adhesion molecules: role of LOX-1. Circ Res 2001; 89(12):1155-60. doi: 10.1161/hh2401.100598 [Crossref] [ Google Scholar]
- Chen M, Masaki T, Sawamura T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther 2002; 95(1):89-100. doi: 10.1016/s0163-7258(02)00236-x [Crossref] [ Google Scholar]
- El-Agamy DS, Nader MA. Attenuation of oxidative stress-induced vascular endothelial dysfunction by thymoquinone. Exp Biol Med (Maywood) 2012; 237(9):1032-8. doi: 10.1258/ebm.2012.012107 [Crossref] [ Google Scholar]
- Idris-Khodja N, Schini-Kerth V. Thymoquinone improves aging-related endothelial dysfunction in the rat mesenteric artery. Naunyn Schmiedebergs Arch Pharmacol 2012; 385(7):749-58. doi: 10.1007/s00210-012-0749-8 [Crossref] [ Google Scholar]
- Alkharfy KM, Ahmad A, Raish M, Vanhoutte PM. Thymoquinone modulates nitric oxide production and improves organ dysfunction of sepsis. Life Sci 2015; 143:131-8. doi: 10.1016/j.lfs.2015.08.007 [Crossref] [ Google Scholar]
- Jha JC, Bose M, Jandeleit-Dahm K. Modulation of oxidative stress in cardiovascular diseases. In: Chakraborti S, Dhalla NS, Dikshit M, Ganguly NK, eds. Modulation of Oxidative Stress in Heart Disease. Singapore: Springer; 2019. p. 237-53. 10.1007/978-981-13-8946-7_10.
- Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (Oxford) 2009; 11(5):373-81. doi: 10.1111/j.1477-2574.2009.00059.x [Crossref] [ Google Scholar]